Transcranial Magnetic Stimulation and Major Depression
Estimulação Magnética Transcraniana e Depressão Maior
Authors:News Author: Batya Swift Yasgur, MA, LSW; CME Author: Laurie Barclay, MD Faculty and Disclosures
CME / ABIM MOC / CE Released: 9/6/2019
Valid for credit through: 9/6/2020
Artigo extraído do MedScape Psychiatry em 15/10/2019
Comentários no final do artigo: Dr Paulo de Mello (Coordenador do GEPECH CEMCO UNIFESP)
Clinical Context
Major depressive disorder (MDD) is highly prevalent, disabling, and associated with resistance to pharmacotherapy and psychotherapy in approximately 20% to 40% of patients. Using electromagnetic induction, repetitive transcranial magnetic stimulation (rTMS) noninvasively modulates brain electrical activity.
For MDD, rTMS is an evidence-based treatment option, but there have previously been no efficacy comparisons between the 2 US Food and Drug Administration (FDA)-approved protocols of rTMS modalities. The goal of this industry-independent, randomized controlled, single-blind trial in patients with MDD was to compare clinical outcomes between the 2 FDA-approved rTMS protocols delivered by H1-coil and the figure-8-coil. The hypothesis tested by this study was that H1-coil HF-rTMS would be more effective than figure-8-coil HF-rTMS because the former directly stimulates larger volumes deeper into the prefrontal cortex, and in earlier trials showed greater remission and response rates.
Study Synopsis and Perspective
Deep rTMS plus standard antidepressant medication is significantly more effective at reducing depression levels in patients with MDD than standard rTMS or stand-alone pharmacotherapy, new research suggests.
In a randomized study, 228 participants with treatment-resistant depression received rTMS using an H1-coil (deep rTMS) plus pharmacotherapy, rTMS using a figure-8-coil (standard rTMS) plus pharmacotherapy, or self-standing pharmacotherapy.
Both rTMS protocols, in conjunction with pharmacotherapy, were superior to treatment with pharmacotherapy alone. In addition, secondary analysis showed significantly greater efficacy of rTMS delivered with the H1-coil vs the figure-8-coil.
Both rTMS modalities were comparably safe. No patients dropped out of the study because of adverse events.
“Although the standard H1-coil protocol uses a smaller number of pulses and has a shorter duration of session, it seems to induce a clinical effect that is at least of the same magnitude, and is in fact likely to be greater” than the figure-8-coil, lead author Igor Filipčić, MD, PhD, from the Psychiatric Hospital “Sveti Ivan” and the School of Medicine, University of Zagreb, Croatia, told Medscape Medical News.
“Our findings suggest an additive and clinically meaningful effect of concurrent rTMS and pharmacotherapy, greater than either treatment alone,” added Dr Filipčić, who is also on the Faculty of Dental Medicine and Health, Josip Juraj Strossmayer University of Osijek, Croatia.
The findings were published in the July issue of the Journal of Psychiatric Research.
Treatment Resistance
“It has been estimated that 20%-40% of patients with MDD do not benefit adequately from available interventions, including pharmacotherapy and psychotherapy,” the authors write. “[rTMS] is a non-invasive treatment method that modulates brain electrical activity by electromagnetic induction. High-frequency rTMS (HF-rTMS) [is] applied at 10-20 Hz to the left dorsolateral prefrontal cortex.”
In previous studies, figure-8-coil HF-rTMS showed efficacy vs sham treatment in improving symptoms of depression in patients with treatment-resistant depression.
A novel form of deep TMS (dTMS) using an H1-coil protocol has been approved by the FDA for treating MDD in adults whose condition does not respond to antidepressant medications in the current episode.
The H1-coil methodology delivers dTMS that targets the PFC bilaterally with preference for the left hemisphere, “where at any depth the model field is higher in the left relative to the right hemisphere,” the investigators write.
“rTMS delivered with the H1-coil…allows noninvasive stimulation of brain regions to a depth that has been estimated to reach approximately 4 cm,” Dr Filipčić said.
There are few data on the safety and efficacy of dTMS. One large randomized trial has been conducted in medication-free adults, but no head-to-head trial has compared the 2 different modalities.
To address this knowledge gap, the researchers evaluated and compared the efficacy and safety of the 2 protocols. They compared both interventions as augmentive in the acute treatment of MDD, and they compared both with self-standing pharmacotherapy.
The previous trial was industry sponsored, Dr Filipčić pointed out.
“It is worth noting that the [current] study is manufacturer independent and free of any commercial incentives to investigators of patients,” he said.
Discontinuation, Remission Rates
Patients with MDD (n=228) were randomly assigned in a 1:1:1 ratio to a 4-week treatment regimen with either H1-coil or figure-8-coil, along with standard pharmacotherapy.
Baseline demographic and clinical characteristics were comparable between the groups:
- H1-coil (n=72): 56.9% women; median age, 50 years; median duration of MDD, 10 years.
- 8-coil (n=75): 45.3% women; median age, 51 years; median duration of MDD, 7 years.
- Control group (n=81): 55.6% women; median age, 53 years; median duration of MDD, 9 years.
At baseline, the medications most frequently used were selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors, followed by other antidepressants and antipsychotics. The percentages at which these medications were used were comparable for all 3 groups.
Roughly 40% of patients in each group were also receiving a benzodiazepine.
Both rTMS protocols were administered 5 days per week for 4 weeks (20 sessions total).
All-cause discontinuation rates were lowest in the 8-coil group (4%) compared with the H1-coil and the control group (9.7% and 11.1%, respectively).
The Hamilton Depression Rating Scale (HAM-D17) mean score was 17 in the H1-coil and 8-coil groups, and 19 in the control group.
The primary outcome was the proportion of patients who achieved remission, which was defined as having a HAM-D17 score of 7 or less.
Favorable Results
The number of patients who achieved remission was higher in the dTMS/H1-coil group (60%; 95% confidence interval [CI], 48%-71%) and the rTMS/8-coil group (43%; 95% CI, 31%-55%) than in the control group (11%; 95% CI, 5%-20%).
However, after adjusting for all preplanned confounders, the remission rate (HAM-D17 of ≤7) was not significantly different between the 2 rTMS modalities, either in the intent-to-treat (ITT) or the per protocol populations.
The odds ratio (OR) for remission in ITT analysis of the H1-coil group vs the 8-coil group was 1.74 (95% CI, 0.79-3.83; P=.17).
In contrast, in the ITT population, patients with baseline moderate/severe depression (HAM-D17 ≥17) in the H1-coil group had significantly higher odds for remission compared with patients in the figure-8-coil group (OR, 4.59; 95% CI, 1.69-12.48; P=.003).
No significant differences were observed in ORs for remission between patients with mild depression (HAM-D17 <7) or other sociodemographic and clinical characteristics.
The response rate, defined as a decrease in HAM-D17 score of at least 50%, was significantly better in both active treatment groups and in the control group.
It was also significantly better in the H1-coil group than in the 8-coil group (67% vs 44%, respectively), after adjustment for all preplanned confounders.
Both HF-rTMS modalities were generally well tolerated. No serious adverse events were reported; the most commonly reported adverse event in both rTMS groups was headache.
The authors note that treatment parameters of rTMS modalities, such as pulse frequency, number of pulses per session, and train duration, were not matched because the study was “pragmatic, with an intent to assess the effectiveness of commonly used FDA-approved protocols in clinical practice.”
However, the results were “favorable compared to short-term efficacy of other treatment approaches, especially considering the high level of treatment resistance of this population,” they write.
Moreover, the treatment delivered by the H1 coil “may be more effective for treatment of MDD severity,” they note.
All members of the healthcare team should be aware of this current research, assess individuals for changes in depressive symptoms and collaborate to consider the benefits of neurostimulation modalities in individuals with depressive disorder.
Clear Clinical Implications
Commenting on the study for Medscape Medical News, Chris Baeken, MD, PhD, associate professor of psychiatry, Ghent University and the Free University, Brussels, Belgium, said the findings have a clear message for clinicians.
“If depressed patients do not or partially respond to the classic antidepressant medication, add-on or better augmentation with these neurostimulation [modalities] may be beneficial to a large group of them, with a slightly better outcome for the H-1 dTMS coils compared to the more commonly used figure-8 coil,” said Dr Baeken, who was not involved with the research.
Dr Filipčić agreed.
“While some questions remain for future studies, clear clinical implications can already be derived from these findings,” he said.
“Firstly, both modalities of rTMS can safely be combined with pharmacotherapy to achieve a higher likelihood of remission; and secondly, both modalities are equally safe and tolerable,” he added.
The study was funded by the Psychiatric Hospital “Sveti Ivan.” The study authors and Dr Baeken have disclosed no relevant financial relationships.
J Psychiatr Res. 2019;114:113-119.[1]
Study Highlights
- Patients with MDD (n=228) were recruited from clinical practice and randomly assigned 1:1:1 to 20 sessions (5 days/week for 4 weeks) of H1-coil or 8-coil as an adjunct to standard-of-care pharmacotherapy, or to standard-of-care pharmacotherapy alone.
- Baseline demographic and clinical characteristics were comparable between the groups.
- Median age was 50 years in the H1-coil group (n=72); 56.9% were women, and median duration of MDD was 10 years.
- In the 8-coil group (n=75), median age was 51 years; 45.3% were women; and median duration of MDD was 7 years.
- In the control group (n=81), median age was 53 years; 55.6% were women; and median duration of MDD was 9 years.
- The 3 groups had similar baseline MDD symptom severity, with mean Hamilton depression rating scale (HAM-D17) score of 17±5.3 in H1-coil, 17±5.4 in 8-coil, and 19±6.1 in the control group.
- All 3 groups had similar proportions of medications used at baseline.
- Selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors were used by the greatest percentages of patients, followed by other antidepressants and antipsychotics; ~40% in each group were also treated with a benzodiazepine.
- The proportion of patients achieving remission, defined as HAM-D17 score of 7 or less at end of treatment at week 4, was the main study endpoint.
- Odds ratio for remission was 1.74 (95% CI, 0.79-3.83) in the H1-coil vs the 8-coil group, which was not statistically significant in the ITT or per protocol analysis, after adjustment for all preplanned confounders.
- However, in the ITT analysis, patients with baseline moderate/severe depression (HAM-D17 ≥17) in the H1-coil group had significantly higher odds for remission than those in the figure-8-coil group (OR, 4.59; 95% CI, 1.69-12.48; P=.003).
- Compared with the control group, both HF-rTMS groups had a significantly greater remission rate (60% [95% CI, 48%-71%] in H1 coil, 43% [95% CI, 31%-55%] in 8-coil, and 11% [95% CI, 5%-20%] in the control group.
- Compared with the 8-coil group, the H1-coil group had a significantly better response (OR, 2.33; 95% CI, 1.04-5.21; P=.04).
- Reductions in HAM-D17 were 59% in the H1-coil, 41% in the 8-coil (P=.048), and 17% in the control group (P<.001 vs H1-coil; P=.003 vs 8-coil).
- Both HF-rTMS groups had similar safety, tolerability, and changes in quality of life, and there were no dropouts for adverse events in either group.
- Headache was the most commonly reported adverse event in both rTMS groups.
- All-cause discontinuation rates were 4% in the 8-coil group, 9.7% in the H1-coil, and 11.1% in the control group.
- On the basis of their findings, the investigators concluded that both FDA-approved HF-rTMS protocols were safe and effective as adjunctive treatments of MDD.
- Although the H1-coil group had a better response rate and greater reduction of depression severity, the 2 rTMS modalities did not differ significantly in remission rate.
- The findings suggest an additive and clinically meaningful effect of rTMS given concurrently with pharmacotherapy, with the combined effect greater than that of either treatment alone.
- This is important in light of the estimated 20% to 40% of patients with MDD who have insufficient benefit from pharmacotherapy and psychotherapy.
- The effects of rTMS were favorable compared to the short-term efficacy of other treatment strategies.
- The primary outcome did not significantly distinguish the 2 TMS protocols, but secondary analyses supported the significantly greater efficacy of the H1-coil vs figure-8-coil, even though the H1-coil protocol uses a smaller number of pulses and sessions of shorter duration.
- The shorter duration of treatment sessions with H1-coil (20 vs 40 minutes in the 8-coil group) may have some advantages in the clinical setting, as the number of patients treated per device may be doubled, and it may be more practical for patients and medical staff.
- Although the standard H1-coil protocol uses shorter sessions and a smaller number of pulses than the 8-coil protocol, its clinical effect is similar and may even be greater, as suggested by the outcomes in this study.
- This FDA-approved form of dTMS is indicated for adults with MDD refractory to antidepressant medications in the current episode.
- The H1-coil targets the PFC bilaterally but the left hemisphere preferentially, in that the model field is higher in the left than in the right hemisphere at any depth, and it noninvasively stimulates brain regions to an estimated depth of ~4 cm.
- Because patients were consecutive, seen for MDD at a public hospital, treated in a regular care setting, and evaluated by blinded raters, the efficacy findings may be comparable to those that would be expected in a real-world clinical setting.
- Study limitations include lack of matching of rTMS modality treatment parameters, such as pulse frequency, number of pulses per session, and train duration, because this was a pragmatic study aiming to evaluate the efficacy of commonly used FDA-approved protocols in clinical practice.
- In addition, patients treated with rTMS modalities were monitored daily, whereas the control group was monitored only at baseline and after 4 weeks of treatment, which might have overestimated the efficacy of both TMS treatments.
- Other limitations include lack of longer-term outcomes and neurocognitive assessments, and that it was a single-center trial, limiting generalizability of the findings.
- The investigators recommend a larger, multisite study to show conclusively the superior efficacy of the H1-coil compared with the figure-8-coil.
Clinical Implications
- Augmentative HF-rTMS treatment for MDD increased response and remission rates compared with pharmacotherapy alone.
- These effects were favorable compared with short-term efficacy of other treatment strategies, especially given the high level of treatment resistance in MDD.
- Implications for the Healthcare Team: H1-coil vs 8-coil had better response rate and reduction of depression severity but similar remission rate, and both rTMS modalities ware equally safe and well tolerated.
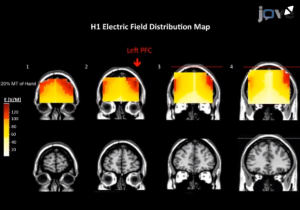 Fig 1: área de estimulação cerebral (F3) com bobina H1.
Fig 1: área de estimulação cerebral (F3) com bobina H1.
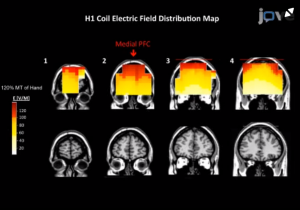 Fig 1: área de estimulação cerebral (Fz) com bobina H1.
Fig 1: área de estimulação cerebral (Fz) com bobina H1.
Comentários:
Este é um estudo controlado, randomizado, cego e bastante interessante (n: 228), o qual se avalia a eficiência na utilização da Estimulação Magnética Transcraniana repetitiva (EMTr) em pacientes com Depressão Maior (Major), combinado ou não ao consumo de psicofármacos sob duas bobinas diferentes, a H1 e a F8. Algumas conclusões deste trabalho merecem ser comentadas. Os resultados evidenciam ligeira vantagem para a bobina H1 em pacientes em situação mais crítica, mas o estudo estatístico não mostrou diferença significativa nos resultados com uma ou com outra bobina, entre a H1 e F8. Ambas evidenciaram significativa melhora no quadro de pacientes deprimidos. Outro resultado bastante interessante foi o fato de que demonstrou-se melhora bastante significativa no score de pacientes deprimidos com resultados desejados insuficientes só com psicofármaco. Isso demonstra que a associação da EMTr com psicofármaco pode ser uma boa estratégia na busca da remissão do Transtorno Monopolar Depressivo Maior em pacientes que fazem uso de psicofármacos cuja remissão não foi alcançada, sobretudo se considerarmos que entre 20 a 40% dos pacientes que fazem uso de psicofármacos não tem uma remissão completa, mesmo que corretamente medicado.
Comentários:
Dr Paulo de Mello
Coordenador do GEPECH CEMCO UNIFESP
Neurociência aplicada à Psicanálise

Leave A Comment